Making the switch to Tritan™ MXF copolyester
NOTE: We appreciate you being part of our TritanMoldIt community! To ensure we are covering the information you need to best serve your customers and advance your business, we invite you to fill out this brief survey. As a thank you, you will have the option to be entered in a drawing for an Amazon Echo Show!
- Read more about Making the switch to Tritan™ MXF copolyester
- Log in or register to post comments
Working together to solve medical device challenges

Molders and brand owners come to us for many different reasons during the developmental journey of a medical device.
• You may have a totally new device and want the best-fit polymer to optimize its performance advantages.
• Your current material may not have the chemical resistance and toughness to meet changing hospital disinfectant protocols and more powerful drugs and carrier solvents.
• Recent regulations may be driving the need for more compliant materials.
Whatever the challenge, working with Eastman lets you access technical expertise and support every step of the way—to help position your device for end-state success.
By collaborating early and often during product development and manufacturing, we can help you work with product engineers, toolmakers, and downstream assembly partners to bring your project to market efficiently and profitably.
Here are a few of the benefits of working together with Eastman:
- Read more about Working together to solve medical device challenges
- Log in or register to post comments
Why should you choose Eastman medical grade polymers?

You want to create medical devices and packaging that ensure patient safety and provide long-lasting reliability. To accomplish that, you have to choose high performance medical grade materials. Manufacturers who specify medical grades of Eastman Tritan™ copolyester not only get access to advanced, high-quality raw materials but also to a strong level of support throughout the regulatory journey to commercialization of a new product.
By specifying Eastman medical grade polymers, you get help with:
By specifying Eastman medical grade polymers, you get help with:
- Biocompatibility
- Sterilization
- Quality systems
- Dedicated regulatory support
- Read more about Why should you choose Eastman medical grade polymers?
- Log in or register to post comments
The effect of electron beam sterilization on Eastman Tritan™

Compared to gamma radiation, e-beam radiation typically costs less due to higher dose rates that reduce the time of exposure at the same target dose. The shorter exposure time also minimizes the oxidation reactions that can occur at the polymer surface, resulting in less effect on resin properties than with gamma radiation.
- Read more about The effect of electron beam sterilization on Eastman Tritan™
- Log in or register to post comments
Overmolding with Tritan

Using overmolded soft-touch materials can provide many functional and decorative benefits to items made from rigid thermoplastics. Eastman Tritan™ copolyester demonstrates exceptional adhesion with commercial grades of TPE. When selecting the TPE you want to use, make sure it is formulated for use with a copolyester substrate.
Consider these factors for part design:
Consider these factors for part design:
- Optimize part and TPE thickness for adhesion and dimensional stability. If the TPE thickness is in excess of the Tritan part thickness, you could see warpage when you remove it from the mold. Use a substrate thickness twice that of the TPE.
- Incorporate mechanical interlocks to improve TPE adhesion and promote part durability. Mechanical interlocks are important for thin TPE layers and very demanding fitness-for-use requirements.
- Read more about Overmolding with Tritan
- Log in or register to post comments
Educating product managers on how plastics affect your brand

The material you choose for your medical devices can have a big impact on your brand’s image. In today’s healthcare environment, not all plastics can withstand exposure to the aggressive disinfectants being used in hospitals. If your device is showing outward signs of suffering from exposure to effects of disinfection, including yellowing, cracking, crazing, or paint peeling, it’s time to reconsider material selection.
- Read more about Educating product managers on how plastics affect your brand
- Log in or register to post comments
Polymer compatibility with oncology drugs
As part of the continued effort to improve cancer treatment, pharmaceutical companies are developing new and improved oncology drugs. However, advanced oncology drugs and 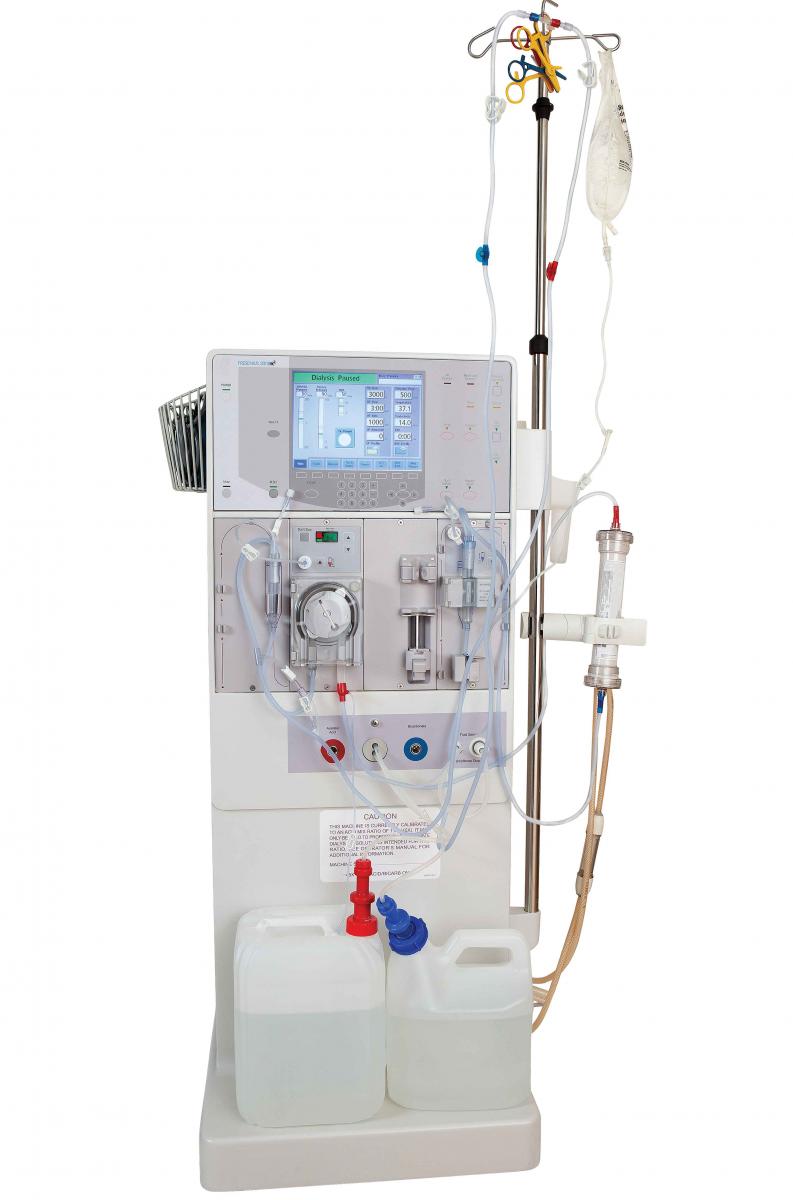 carrier solvents challenge the chemical resistance of polymers used in delivery devices. Such conditions can prevent devices from working properly or cause them to fail prematurely. When there is a pattern of compromised device performance or life cycle, regulatory agencies may tell manufacturers to stop using certain materials to protect patient safety.
carrier solvents challenge the chemical resistance of polymers used in delivery devices. Such conditions can prevent devices from working properly or cause them to fail prematurely. When there is a pattern of compromised device performance or life cycle, regulatory agencies may tell manufacturers to stop using certain materials to protect patient safety.
 carrier solvents challenge the chemical resistance of polymers used in delivery devices. Such conditions can prevent devices from working properly or cause them to fail prematurely. When there is a pattern of compromised device performance or life cycle, regulatory agencies may tell manufacturers to stop using certain materials to protect patient safety.
carrier solvents challenge the chemical resistance of polymers used in delivery devices. Such conditions can prevent devices from working properly or cause them to fail prematurely. When there is a pattern of compromised device performance or life cycle, regulatory agencies may tell manufacturers to stop using certain materials to protect patient safety.
- Read more about Polymer compatibility with oncology drugs
- Log in or register to post comments
Better bonds between polymers and adhesives
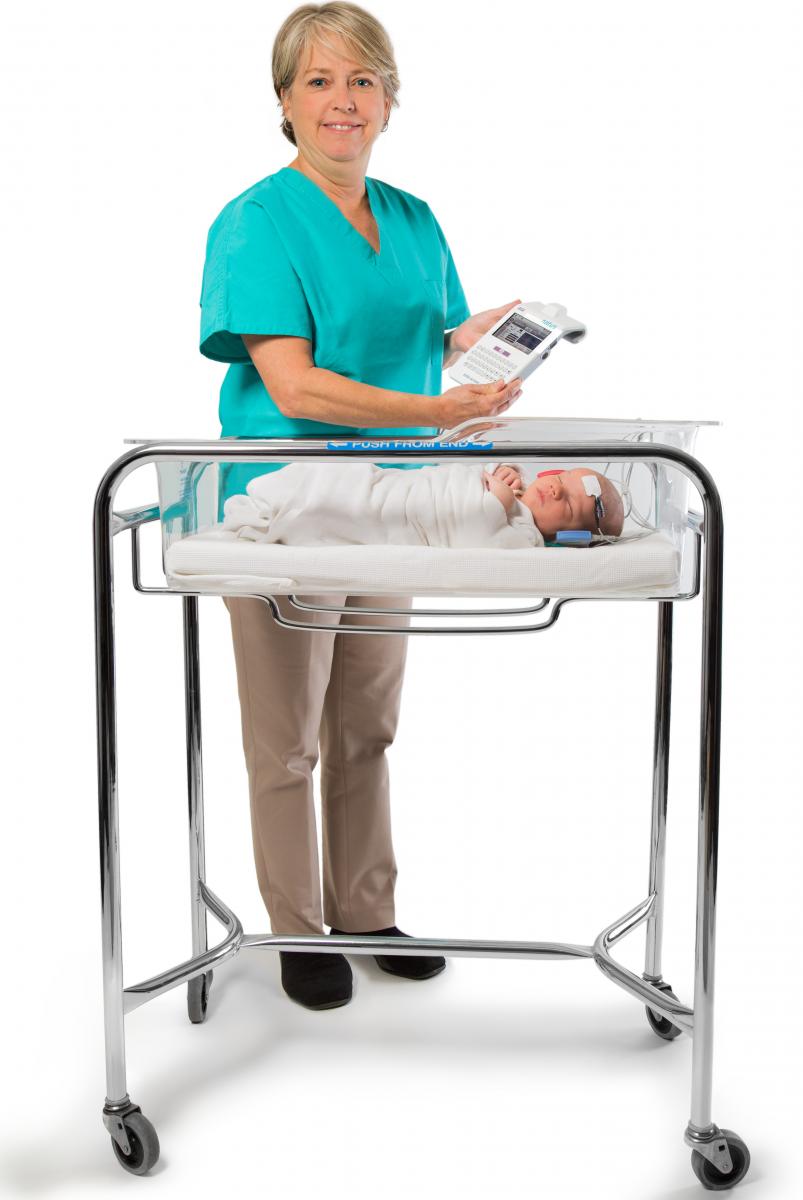 We often receive requests from medical device developers and original equipment manufacturers for guidance on the best adhesives to use with Eastman TritanTM copolyester. To help customers achieve the best adhesive solutions, we partnered with Henkel Corporation to test various resins and adhesives for use in medical devices.
We often receive requests from medical device developers and original equipment manufacturers for guidance on the best adhesives to use with Eastman TritanTM copolyester. To help customers achieve the best adhesive solutions, we partnered with Henkel Corporation to test various resins and adhesives for use in medical devices.Henkel’s LOCTITE® adhesive continues to be tested at the industry’s most comprehensive ISO 10993 biocompatibility standards. Eastman looked to determine which resins and adhesives, when used with Tritan, could optimize a manufacturer’s assembly process. Results showed that the use of Tritan and LOCTITE together created superior results, including improved
curing to increased flexibility.
- Read more about Better bonds between polymers and adhesives
- Log in or register to post comments
Creating strong bonds with LSR technology and Tritan
Medical devices and housings are getting a surge of chemical resistance and impact strength thanks to the integration of new liquid silicone rubber (LSR) technology with medical grades of Eastman Tritan™ copolyester. Momentive’s Silopren LSR 47×9 series provides strong in-mold adhesion with Tritan—without the need for primers.
Clear and opaque grades of Tritan have a lower Tg and require a lower processing temperature than other engineering polymers. Because Silopren LSR 47×9 can cure rapidly at relatively low temperatures, it’s possible to achieve optimal functional performance and efficient processing with Tritan.
This combination is ideal for applications that require properties like handling comfort, waterproofing, durability, and aging stability. Incorporating LSR technology enhances the advantages of Tritan, which include:
Clear and opaque grades of Tritan have a lower Tg and require a lower processing temperature than other engineering polymers. Because Silopren LSR 47×9 can cure rapidly at relatively low temperatures, it’s possible to achieve optimal functional performance and efficient processing with Tritan.
This combination is ideal for applications that require properties like handling comfort, waterproofing, durability, and aging stability. Incorporating LSR technology enhances the advantages of Tritan, which include:
- Outstanding chemical resistance
- Excellent impact strength and durability
- Made without bisphenol A (BPA) and halogens
- Superior noise-damping characteristics
- Read more about Creating strong bonds with LSR technology and Tritan
- Log in or register to post comments
Four steps for testing housing material performance
This simple, easily-repeatable test can help predict the reliability of a material after exposure to harsh cleaners and drugs commonly used in hospital settings. The method uses a 1.5% constant strain jig together with wet patches for applying chemical reagents. Here’s how it works:
- Select the appropriate jig.
- Load flex bars onto jig.
- Apply chemicals to the flex bars.
- Perform reverse side impact test.
- Read more about Four steps for testing housing material performance
- Log in or register to post comments




 Close
Close


